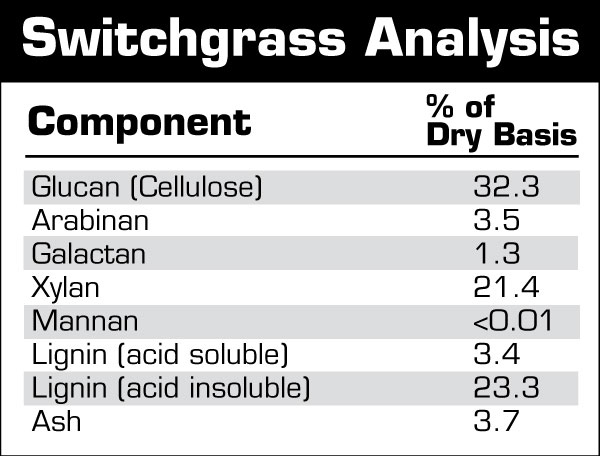
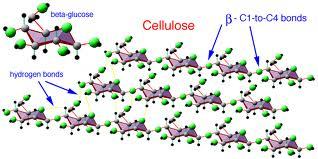
Demystifying Cellulose



October 11, 2013
BY Scott Mowrey
Advertisement
Advertisement
Related Stories
The USDA expanded its estimate for 2023-’24 corn use in ethanol to 5.45 billion bushels in its latest WASDE report, released May 10. The agency currently expects the volume of corn that goes to ethanol production to remain at that level for 2024-’25.
The USDA’s Agricultural Research Service, the University of Nebraska–Lincoln, and Nebraska Innovation Campus on May 6 celebrated the groundbreaking of the National Center for Resilient and Regenerative Precision Agriculture.
Brazil’s 2024-’25 sugarcane harvest season kicked off on April 1. UNICA, the country’s sugarcane industry association, said sugarcane processing and ethanol production were both up during the first two-weeks of the harvest season.
The USDA recently released its Grain Crushings and Co-Products Production Report for March, reporting that corn use for fuel ethanol production in February was up when compared to both the previous month and February 2023.
Indigo Ag and Red Trail Energy have announced a collaboration to source low carbon intensity (CI) corn to support farmers using sustainable practices and benefit from emerging clean fuels market tax credit programs.




