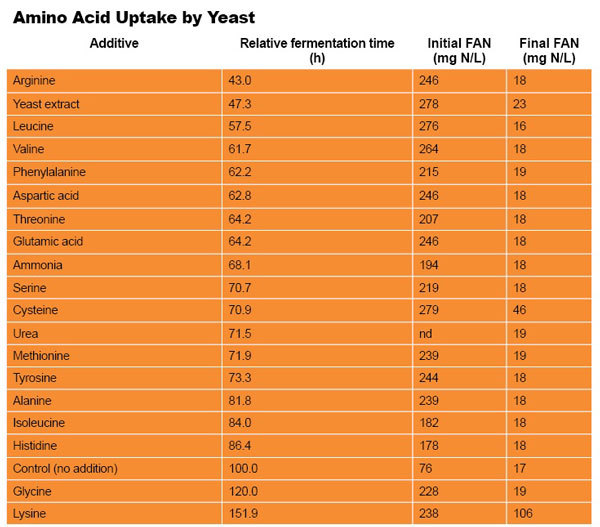
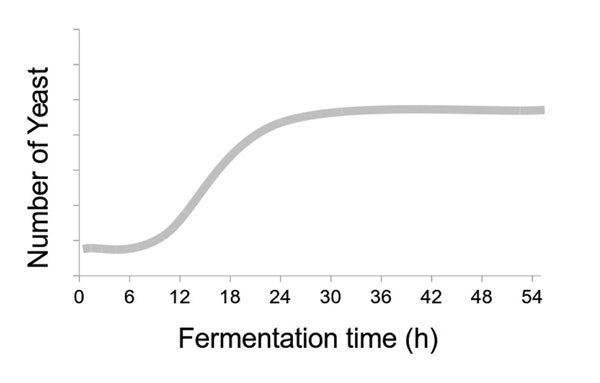
Protease Use for FAN as Urea Substitute Poses Challenges


May 19, 2017
BY Dennis Bayrock
Advertisement
Advertisement
Related Stories
Nearly 1.91 billion RINs were generated under the Renewable Fuel Standard in March, down from 1.92 billion generated during the same month of 2023, according to data released by the U.S. EPA on April 18.
The U.S. EPA on April 18 released updated small refinery exemption (SRE) data, reporting that two additional SRE petitions have been filed under the Renewable Fuel Standard in the past month. A total of 38 SRE petitions are now pending.
U.S. fuel ethanol production was down 7% the week ending April 12, according to data released by the U.S. Energy Information Administration on April 17. Stocks of fuel ethanol were down slightly and exports expanded by more than 12%.
A record volume of sugarcane was processed during Brazil’s recently completed 2023-’24 harvest season, according to UNICA, the Brazilian sugarcane industry association. Ethanol production also set a new record, with a significant boost from corn.
Marquis and United Cooperative on April 16 announced the sale of Marquis’ 100 MMgy Necedah, Wisconsin, ethanol facility to United Cooperative. The facility will operate under the new name of United Energy Necedah LLC.





