Ethanol: The Renewable Naphtha

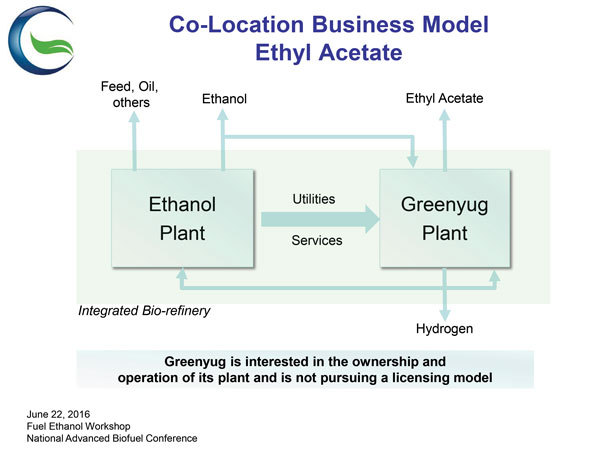

PHOTO: GREENYUG
May 29, 2018
BY Luca Zullo
Advertisement
Advertisement
Related Stories
U.S. fuel ethanol production fell by 3% the week ending April 19, according to data released by the U.S. Energy Information Administration on April 24. Stocks of fuel ethanol were down 1% and exports fell by 23%.
Vertimass on April 23 announced that the U.S. EPA has approved registration for blending up to 20% of Vertimass green gasoline with conventional gasoline. This new renewable gasoline product, VertiGas20, is made from renewable ethanol.
The USDA on April 23 awarded more than $43 million in grants through the Higher Blends Infrastructure Incentive Program to support projects that will increase the availability of domestic biofuels in 15 states.
Indigo Ag and Red Trail Energy have announced a collaboration to source low carbon intensity (CI) corn to support farmers using sustainable practices and benefit from emerging clean fuels market tax credit programs.
CoBank is predicting a positive outlook for ethanol in 2024 as plants capitalize on lower corn prices and improved margins, according to the company’s latest Quarterly Research Report, released April 11.




