Bits of the Batch


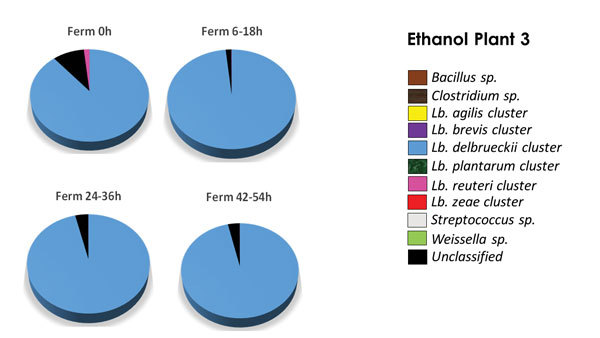
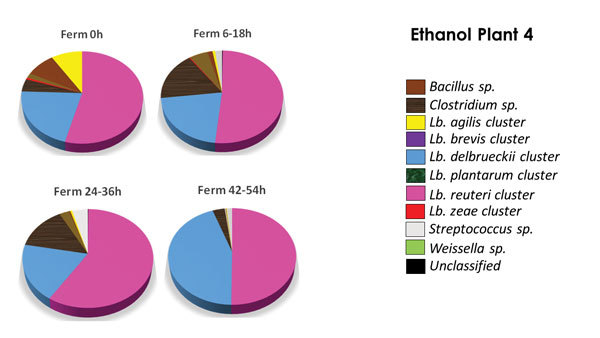

PHOTO: LALLEMAND BIOFUELS & DISTILLED SPIRITS
March 27, 2020
BY Lisa Gibson
Advertisement
Advertisement
Related Stories
EIA maintains forecast for 2024 and 2025 ethanol production, increases forecast for 2025 ethanol blending
The U.S. Energy Information Administration maintained its forecast for 2024 and 2025 fuel ethanol production in its latest Short-Term Energy Outlook, released May 7. The agency also maintained its forecast for 2024 ethanol blending but increased its forecast for 2025 blending.
Alto Ingredients Inc. on May 6 reported that its Q1 earnings were negatively impacted by low crush margins and inclement weather, but that high-quality alcohol sales contributed to improved gross profit.
Novonesis, a company formed via the January 2023 merger of Novozymes and Denmark-based bioscience company Chr. Hansen, released first quarter financial results on May 3, reporting strong results for its Bioenergy segment.
In August, maintenance personnel will gather at the Team M3 event in Sioux City, Iowa, to share knowledge, help each other maximize uptime and solve production problems.
When it comes to distillers corn oil (DCO) generated as a coproduct of ethanol production, every drop adds to the overall profitability of the operation.