The Basics of Ethanol Plant Laboratory Quality Management


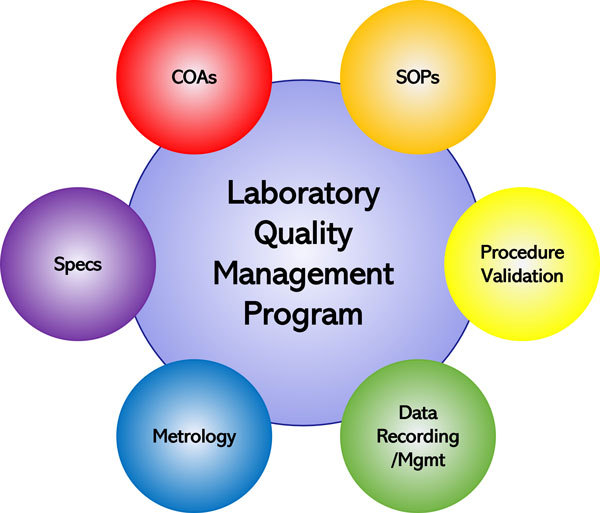

PHOTO: PHIBRO ETHANOL
October 8, 2021
BY Tom Bryan
Advertisement
Advertisement
Related Stories
U.S. fuel ethanol production was down 7% the week ending April 12, according to data released by the U.S. Energy Information Administration on April 17. Stocks of fuel ethanol were down slightly and exports expanded by more than 12%.
A record volume of sugarcane was processed during Brazil’s recently completed 2023-’24 harvest season, according to UNICA, the Brazilian sugarcane industry association. Ethanol production also set a new record, with a significant boost from corn.
The ethanol and ethanol co-product industry continues to be a main economic driver in Nebraska, producing near-historic averages in 2020, despite lower ethanol prices and COVID-related production issues, according to a new study.
U.S. fuel ethanol production fell by nearly 2% the week ending April 5, according to data released by the U.S. Energy Information Administration on April 10. Ethanol stocks were down nearly 1% and exports expanded by 120%.
Trident Automation leverages its industry experience and innovation to help ethanol producers maintain and install distributed control systems.





