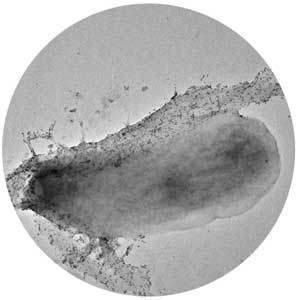
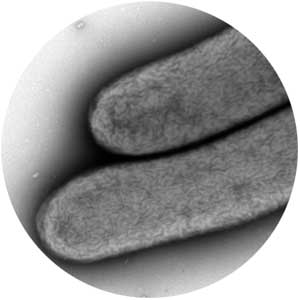
Researchers create cellulose-producing microbe

June 2, 2008
BY Dave Nilles
Advertisement
Advertisement
Upcoming Events
2024 International Fuel Ethanol Workshop & Expo
June 10-12, 2024
MINNEAPOLIS CONVENTION CENTER | MINNEAPOLIS,MINNESOTA
Now in its 40th year, the FEW provides the ethanol industry with cutting-edge content and unparalleled networking opportunities in a dynamic business-to-business environment. As the largest, longest running ethanol conference in the world, the FEW is renowned for its superb programming—powered by Ethanol Producer Magazine —that maintains a strong focus on commercial-scale ethanol production, new technology, and near-term research and development. The event draws more than 2,000 people from over 31 countries and from nearly every ethanol plant in the United States and Canada.View More
Biodiesel Summit: Sustainable Aviation Fuel & Renewable Diesel
June 10-12, 2024
MINNEAPOLIS CONVENTION CENTER | MINNEAPOLIS,MINNESOTA
The Biodiesel Summit: Sustainable Aviation Fuel & Renewable Diesel is a forum designed for biodiesel and renewable diesel producers to learn about cutting-edge process technologies, new techniques and equipment to optimize existing production, and efficiencies to save money while increasing throughput and fuel quality. View More
Carbon Capture & Storage Summit
June 10-12, 2024
MINNEAPOLIS CONVENTION CENTER | MINNEAPOLIS,MINNESOTA
Capturing and storing carbon dioxide in underground wells has the potential to become the most consequential technological deployment in the history of the broader biofuels industry. Deploying effective carbon capture and storage at biofuels plants will cement ethanol and biodiesel as the lowest carbon liquid fuels commercially available in the marketplace. The Carbon Capture & Storage Summit will offer attendees a comprehensive look at the economics of carbon capture and storage, the infrastructure required to make it possible and the financial and marketplace impacts to participating producers.View More
North American SAF Conference & Expo
September 11-13, 2024
SAINT PAUL RIVERCENTRE | SAINT PAUL,MINNESOTA
Taking place September 11-13, 2024 in Saint Paul, Minnesota, the North American SAF Conference & Expo, produced by SAF Magazine, in collaboration with the Commercial Aviation Alternative Fuels Initiative (CAAFI) will showcase the latest strategies for aviation fuel decarbonization, solutions for key industry challenges, and highlight the current opportunities for airlines, corporations and fuel producers.View More
2025 International Biomass Conference & Expo
March 18-20, 2025
COBB GALLERIA CENTRE | ATLANTA,GEORGIA
Now in its 18th year, the International Biomass Conference & Expo is expected to bring together more than 900 attendees, 160 exhibitors and 65 speakers from more than 25 countries. It is the largest gathering of biomass professionals and academics in the world. The conference provides relevant content and unparalleled networking opportunities in a dynamic business-to-business environment. In addition to abundant networking opportunities, the largest biomass conference in the world is renowned for its outstanding programming—powered by Biomass Magazine–that maintains a strong focus on commercial-scale biomass production, new technology, and near-term research and development. Join us at the International Biomass Conference & Expo as we enter this new and exciting era in biomass energy.View More
